- Index
- Ampere-hour
- Ampere-hours
- Brand
- Type
- Agm (61)
- Agm Battery (12)
- Agm Deep Cycle (16)
- Batterie (51)
- Batteries (41)
- Battery (188)
- Battery Cover (19)
- Calcium Battery (22)
- Cycle Profond (15)
- Deep Cycle (12)
- Décharge Lente (37)
- Décharge Lente (22)
- Internal (10)
- Lead Acid Battery (25)
- Lead-acid Battery (20)
- Lithium Battery (24)
- Notebook, Laptop (15)
- Panneau Solaire (11)
- Sla Battery (11)
- Solar Panel (9)
- Other (3583)
- Vehicle Type
- Voltage
HOT CONTEC PM50 Patient Monitor Vital Signs NIBP SPO2 Pulse Oximeter New

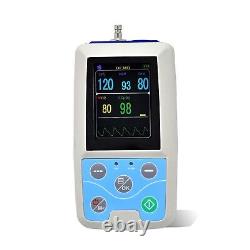

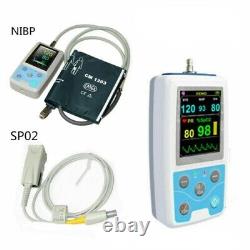










24 hours multifunctional patient monitor, SPO2/NIBP/Pulse Rate PM50. PM50 is a multi-functional patient monitor that can monitor BP and SpO2 at the same time. Realizing long time monitoring of dynamic blood pressure, the device is widely applicable to hospital wards, community clinics and other medical institutions. Compact and portable, easy to use. Double working modes, monitoring function and 24 hours ambulatory NIBP measurement function can be flexibly set.
Long time monitoring BP and SpO2, displaying value of BP, SpO2 and PR. NIBP and SpO2 data Record for large capacity. With friendly user interface, the user can see list menu and review measurement results.
The device can display low power information, alarm information, error information and time information richly. Parameter alarms function is optional. Patient information can be flexibly set.
PC software can achieve data review, analysis measurement results, seeing trend, printing reports and other functions. Data can be transmitted between device and computer, such as upload patient information, download measurement data. Function of master device software.
Set patient information through PC software. Up to 1000 patients cases can be edited and supervised. The time segment of dealing with the patient's NIBP data is 48 hours.
Connect the device by USB interface. Can upload patient information data collection project and download collection data.
Can display scoop-shape trend graph, filling-type trend graph, histogram, pie chart, correlation line graph. Can edit every piece of data, and add annotation to it. Can edit basic information, doctor's advice information, NIBP and SpO2 status instruction, current medicine-taken information, etc. Support print preview, print the report.
Measurement Mode: The upper arm measure.
Automatic Measurement Interval: 5.10.15.20.30.45.60.90.120 minutes. Increasing pressure mode: force pump increases pressure automatically. Reducing pressure mode: self-motion ladder reducing pressure mode.
Alarm parameter: SYS / DIA. Accuracy: 70-100%, ±2%, below 70% unspecified. Error in Weak Filling Condition: SpO2 and pulse rate can be shown correctly when pulse-filling ratio is 0.4%. SpO2 error is ±4%, pulse rate error is ±2 bpm or ±2% (select larger). Resistance to surrounding light: The deviation between the value measured in the condition of man-made light or indoor natural light and that of darkroom is less than ±1%.
Accuracy: ±2 bpm or ±2% (select larger). 2.4 TFT colour LCD. Power: DC 3V (Two "AA", 1.5V Alkali Battery). Product safety type: Type BF application part (Internally powered, defibrillator protected). PC software to be download from the website. Dimension: 1286936 mm (No including Packing).Altitude: -500 to 4,600m (-1,600 to 15,000ft). Altitude: -500 to 13,100m (-1,600 to 43,000ft). Specific EMC, climate, mechanical environment: The patient monitor after packaged should be stored in a well-ventilated room with no corrosive gas.
Strong shock, vibration and snow and rain should be avoided during transportation. Food and Drug Administration and state and local regulatory agencies.The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 197923, and certified by FDA of United States and CE, TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved. These charges are buyers' responsibility.
ECG / ECG holter EEG / EEG Holter B-ultrasound. Patient Monitor Fetal doppler Fetal Monitor. Spo2 Monitor Stethoscope Medical Image...
We are look for partners in the world now! Do you want to become our agent in your country? Mary # hotmail dot com.
